| pH Control
Introduction
Most process plants generate a wastewater effluent
that must be neutralized prior to discharge or reuse.
Consequently, pH control is needed in just about every
process plant, and yet a large percentage of pH loops
perform poorly. Results are inferior product quality,
environmental pollution, and material waste. With ever
increasing pressure to improve plant efficiency and
tighter regulations in environmental protection, effective
and continuous pH control is highly desirable.
However, implementing a pH system is like putting a
puzzle together. It will only work when all the components
are in place. The pH puzzle includes effective pH probes,
actuators, and controllers. While various pH probes
and actuators for pH control are available, commercial
adaptive pH controllers are still in demand. The challenge
is to provide a controller that is able to deal with
large nonlinear gain changes in the pH loop. It will
be useful for not only wastewater neutralization, but
also chemical concentration control, since concentration
is a key quality variable.
Solution - Why is pH Control Difficult?
A strong-acid-strong-base pH process is highly
nonlinear. The pH value versus the reagent flow has
a logarithmic relationship. Away from neutrality, the
process gain is relatively small. Near neutrality where
pH=7, the process gain can be a few thousand times higher.
It is impossible for a fixed controller like PID to
effectively control this process.
In practice, most pH loops are in a “bang-bang”
type of control with pumps cycling on and off, which
causes large oscillations. Since acid and caustic neutralize
each other, over-dosing acid and caustic is prohibitively
expensive. Statistics show that a poorly controlled
pH process can cost tens of thousands of dollars in
chemical usage each month, not counting the penalties
imposed by violating EPA or local government discharge
codes.
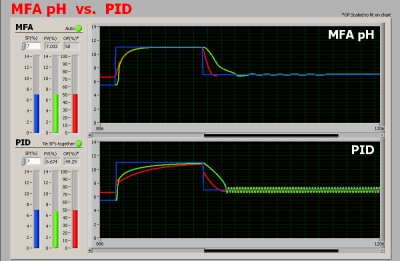
MFA pH Controller vs. PID Controller
The following trends show the MFA
pH controller (top) is able to control
the pH in its full range when the pH setpoint is changed
from 7 to 12 and back to 7. PID (bottom) is either sluggish
or oscillating.
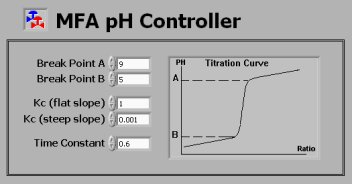
MFA pH Controller Configuration
As shown in the following bitmap, one can easily
enter Break Point A and B to define the estimated shape
of the titration curve of the pH process. Then the MFA
controller gain Kc for the flat portion and steep slope
can be entered. For example, the controller gain for
the flat portion is set to1 for a strong-acid-strong-base
pH process. The estimated gain for the steep slope can
then be set to 0.001, which is 1000 times smaller. Due
to the adaptive capability of the MFA
pH controller, the shape of the titration
curve does not have to be accurately estimated, and,
in actual applications, the shape can vary in real-time.
Lastly, the flow rate and the pH value of the inflows
may vary significantly, and even with these large disturbances,
the MFA
pH controller will be effective.
pH Control
The MFA pH Controller is specially designed
to control pH value for continuous water neutralization.
A special Anti-delay
MFA pH controller can effectively control
pH processes with large time delays.
MFA
pH control users include the following
companies:
Rohm Haas, Shell Oil, PetroBras, Atofino, McDermott
International, Chiron, Unilever, PetroChina, Akzo Nobel,
Morningstar, Sinopec and Baosteel.
To read more about implementations of CyboSoft’s
MFA pH solutions, click on the following case studies:
Model-Free
Adaptive Control on pH Loops - Rohm and Haas
Model-Free
Adaptive Control on Wastewater Neutralization - Chiron
MFA
Control and Optimization of Oil Recovery Boilers - PetroChina
• View
controller video
.9MB ( Macromedia
Shockwave plug-in required )
|